Engineered for Complex, High-Velocity Trials
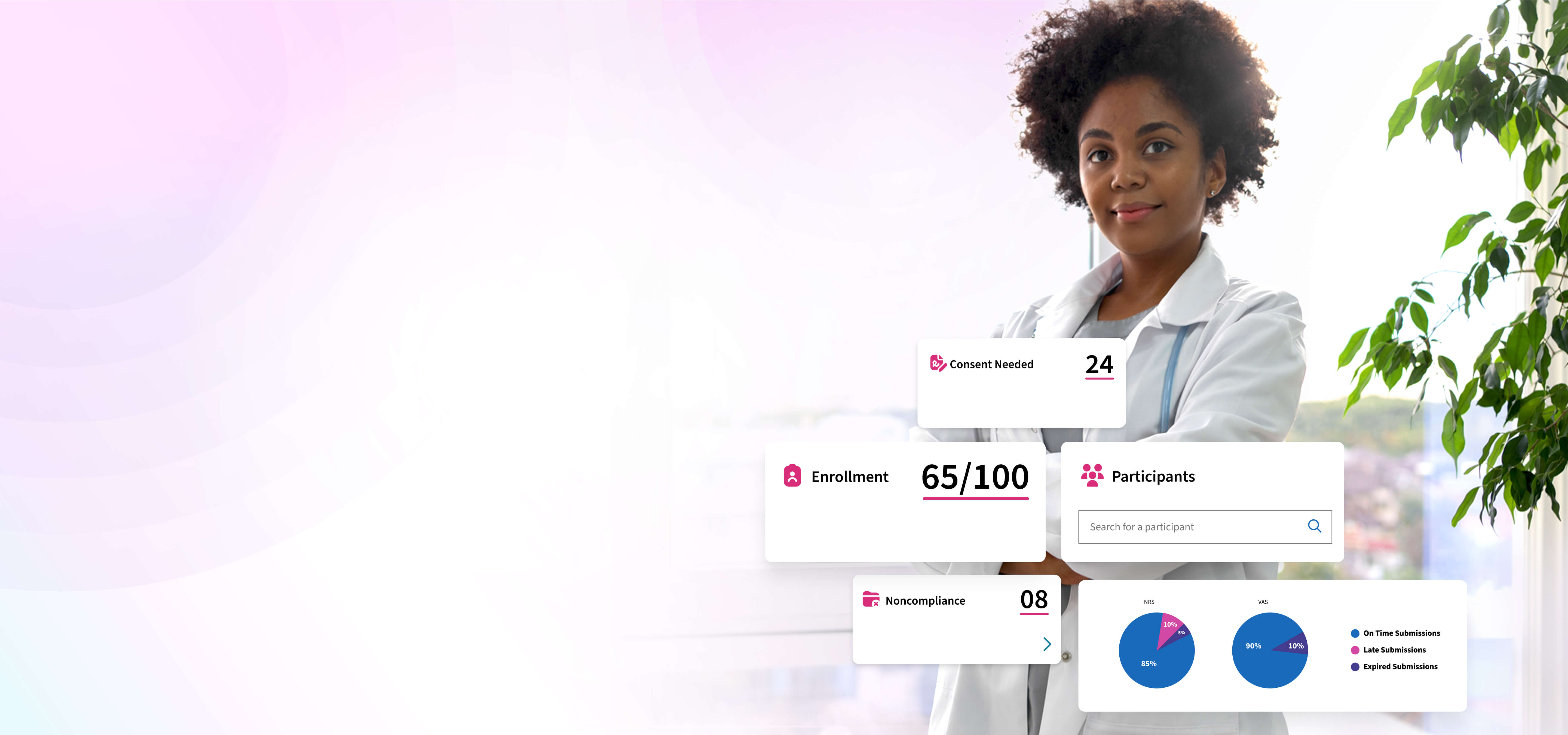
eConsent with AI-Driven Identity Verification
With eConsent and ID verification, the consent process is fully automated, cutting site workload, lowering risk, and reducing participant drop-off, which enables faster, compliant onboarding for even the most time-critical studies.
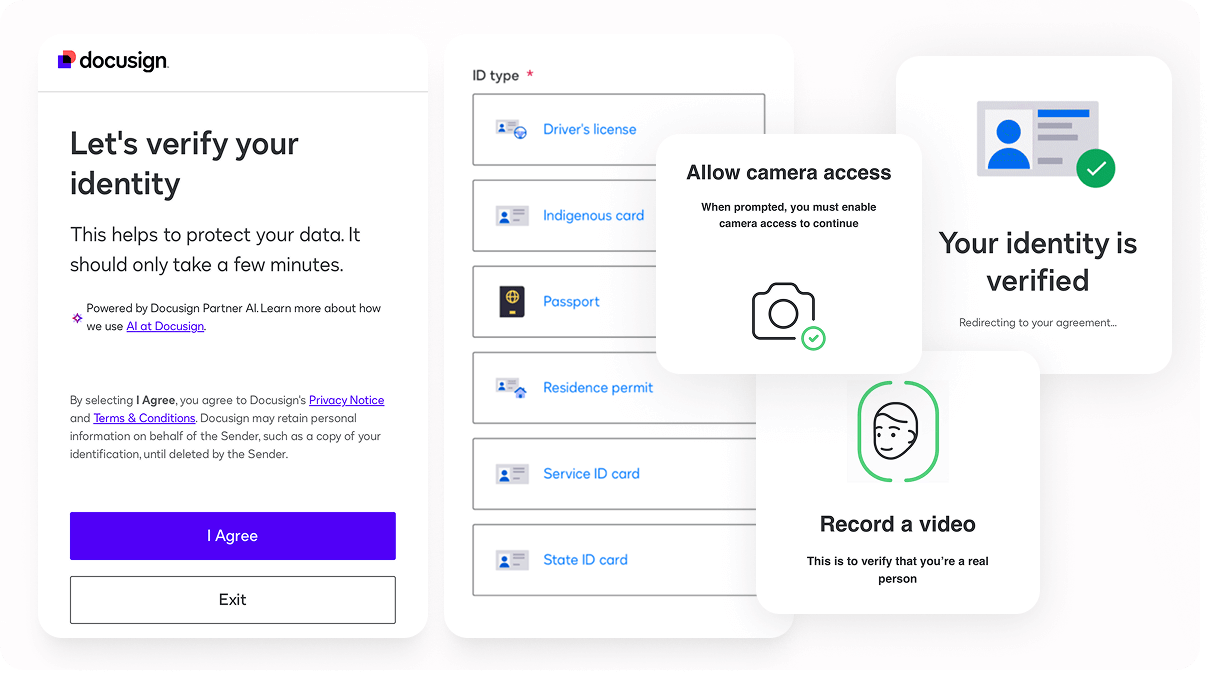
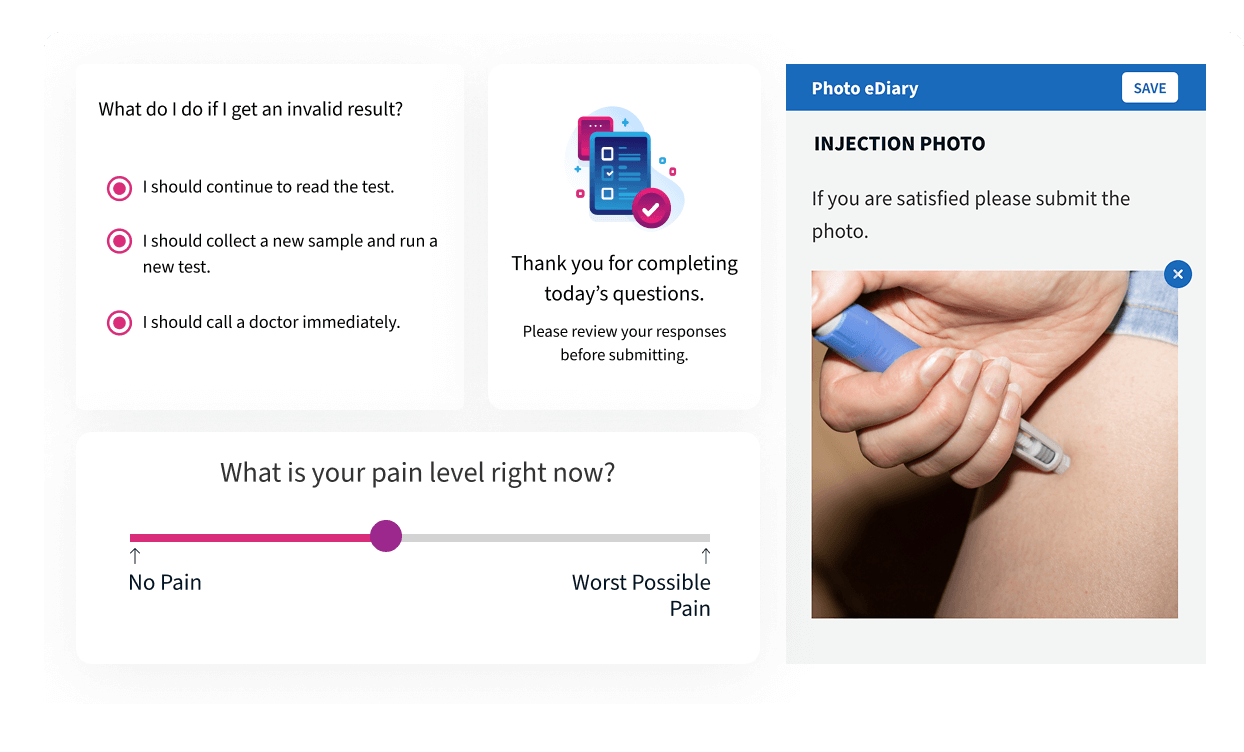
eCOA
Alethium’s event-driven architecture powers intelligent eCOA delivery with scoring-based assessments, point-of-care PROs, and secure photo and document capture. Every study receives the exact configuration needed to meet complex protocol demands with accuracy and speed.
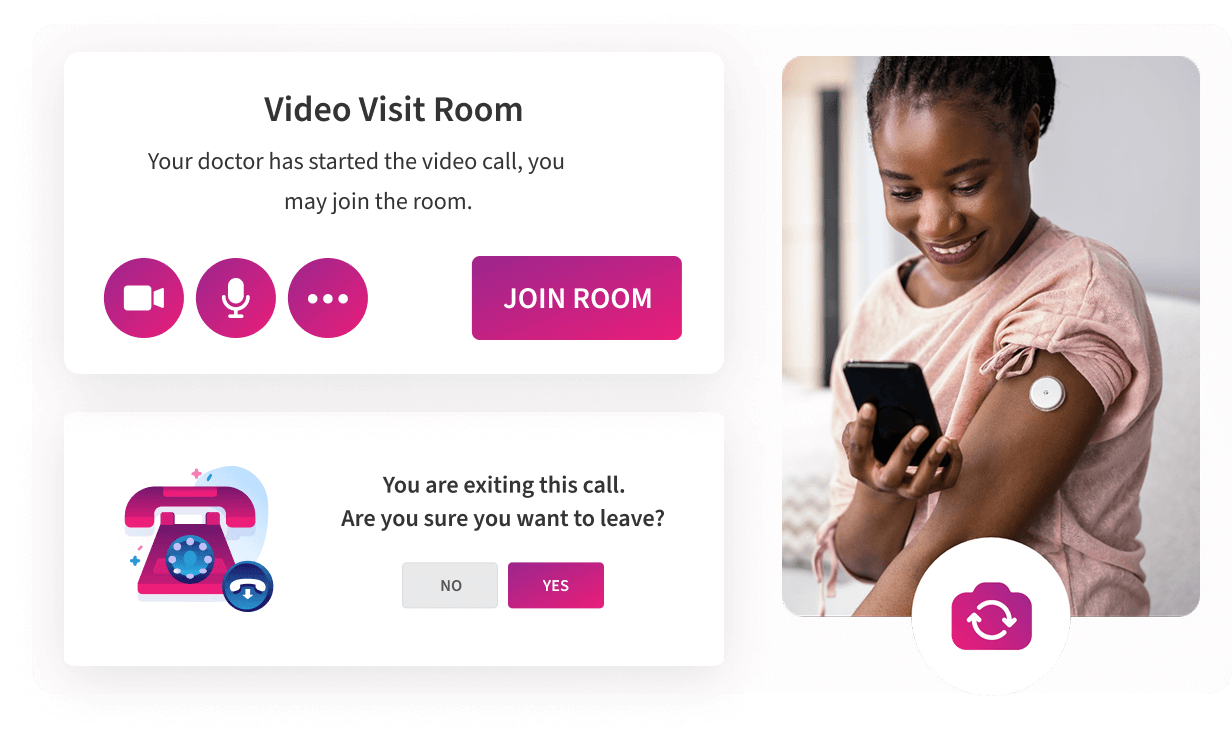
Televisits
Secure televisits turn any device into a compliant data-capture point, simplifying decentralized and hybrid trials, cutting site workload, and delivering real-time evidence for faster, more reliable outcomes.
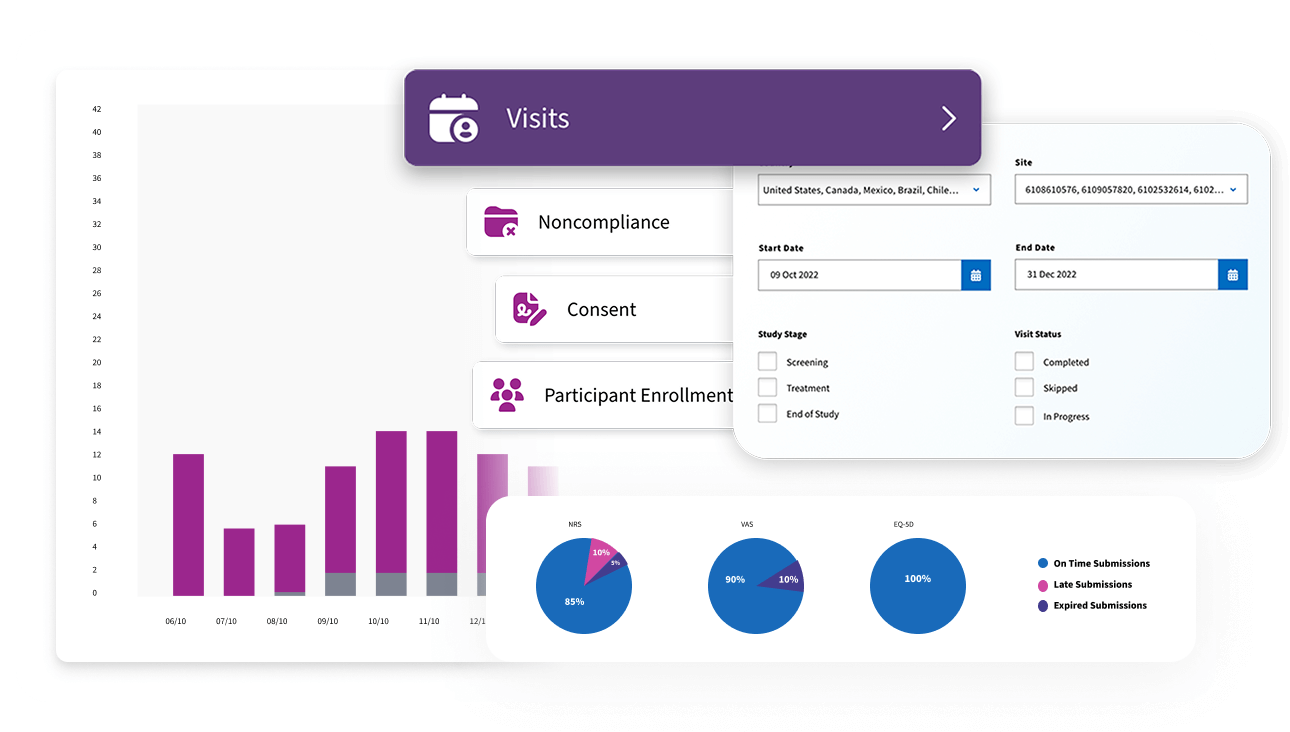
Advanced Analytics
Our analytics turn trial data into actionable insights, sourcing information directly from the event-driven architecture and audit log. Every report and custom dashboard provides verified, reliable study activity for confident decision-making.
Proven Experience, Exceptional Delivery
Since 2008, clients have relied on us to navigate their most demanding challenges—and we’ve delivered through expertise, trust, and meticulous care. Our long-standing team functions with uncommon cohesion, enabling streamlined communication and consistently exceptional outcomes.
Broad Therapeutic Domains
From vaccines to groundbreaking research in Multiple Sclerosis, Oncology, NASH, Chikungunya, Cardiology, Vaccine/Infectious Diseases, Hepatitis B, and Ophthalmology, Alethium has tackled some of the toughest trials for the world’s leading CROs and sponsors.
Early to Late Stage Trials
Our unified, event-driven CDMS and RBQM foundation ensure the precision required in Phase I, the speed and coordination demanded in Phase II, the scale and multilingual complexity of global Phase III programs, and the reliability essential for Phase IV real-world evidence and follow-up studies.
Proven Scale
Alethium has supported more than 250,000 participants through complex U.S. and international trials. Our unified CDMS and automation-driven workflows deliver the control and insight required to manage global coordination at scale.
DCT and Hybrid Success
Alethium has extensive experience executing multi-arm decentralized and hybrid trials, applying an event-driven CDMS and BDD-defined workflows to manage parallel cohorts, remote procedures, and complex protocol pathways. Our architecture keeps each arm synchronized through unified data capture, automated logistics, and continuous RBQM oversight.
Who We Serve
We enable emerging and mid-sized biopharma companies and CROs to meet the same regulatory standards as industry leaders while operating with smaller teams and tighter budgets. By combining an automated CDMS framework with integrated CRO services, we reduce operational burden, shorten timelines, mitigate risk and lower costs.


Risk Mitigation
Alethium’s Risk-Based Quality Management (RBQM) identifies and mitigates risk at every stage of the study. Through automated detection, targeted workflows, and continuous human oversight, Alethium provided proactive quality management throughout every study stage. Our RBQM process delivers emerging biopharmas and CROs greater control, fewer deviations, and the operational clarity to execute even the most complex studies with Alethium.
Clinical Trials
Without Compromise
Transform complex clinical research with intelligent data management that turns obstacles into opportunities. Our platform delivers precision, speed, and clarity at every stage of your trial.
Architecture Is Our Guarantee
Alethium’s digital-first CDMS, with fully integrated CRO services, is engineered with compliance, automation, risk management, and speed at its core.
Event-Driven Audit Log
Our CDMS is built around an event-driven audit log that does more than record changes—it triggers actions and enforces workflows. Each PII record is encrypted individually at rest, delivering enterprise-grade security and a clear, verifiable trail for audits and investigations.
Automation Engine
Alethium’s Automation Engine ensures success for even the most complex, time-sensitive trials. It automates eConsent, data collection, notifications, overnight logistics, alerts, reimbursements, and more. Built on Behavior-Driven Development (BDD) principles, the engine ensures that all CDMS and integrated CRO services execute with precision—delivering unprecedented speed, compliance, and safety.
Encryption at Rest
Each record is encrypted at rest with its own unique key. If a participant withdraws or a study closes, the corresponding encryption keys are deleted, rendering all PII data permanently inaccessible.
Digital-First Design
Guided by a Jobs-to-Be-Done (JTBD) strategy, we build digital-first solutions that address real clinical challenges rather than replicating paper-based workflows. By rethinking how software can eliminate friction and drive measurable outcomes, we deliver faster, more reliable results for Sponsors and CROs.
Integrated Services
Our event-driven architecture seamlessly links the CDMS with any CRO’s systems and services, so actions happen automatically—not manually. The result is instant orchestration, a single-source audit log, real-time analytics, and site teams free to focus on patients instead of paperwork. The outcome: lower costs, less operational friction, and stronger risk mitigation.
Living Documentation
Using Behavior-Driven Development (BDD), we translate complex study protocols into living documentation written in plain language. Sponsors, CROs, and Alethium operate from a shared rule set continuously validated through automated testing. The result: less ambiguity, less rework, and the regulatory-grade accuracy needed to run complex studies with confidence.
Compliance at the Core
Alethium’s CDMS aligns with the world’s most rigorous standards:

HIPAA & GDPR
Per-record encryption and least-privilege access.

SOC 2
Independently audited to validate security, availability, and confidentiality.

21 CFR Part 11
Immutable audit log, electronic signatures, versioning, and role-based access controls.

ICH E6
Risk-based architecture, validated SOPs, and structured processes.
Schedule Your Personalized Demo
We listen to your priorities and focus on the risks that matter most to you. Every demo is tailored to your challenges.
FAQs
Alethium’s digital-first CDMS is built on an event-driven audit log and deep automation. Software and services operate within a single, unified platform—replacing handoffs with seamless integration. The result: reduced risk, accelerated timelines, and immutable evidence for every action, from patient onboarding through regulatory submission.
Compliance is engineered into the architecture: immutable audit log, electronic signatures, versioning, and role-based access (21 CFR Part 11); risk-based processes and validated SOPs (ICH E6); per-record encryption and least-privilege access (HIPAA/GDPR); and independent audits validating security, availability, and confidentiality (SOC 2).
Yes. Sponsors, CROs, and site staff can run complex decentralized or hybrid trials with confidence. Automation and integration ensure consistent workflows—from onboarding and data capture to analytics and submissions—enhancing participant protection, reducing site workload, and shortening timelines while maintaining full compliance.
Alethium’s event-driven architecture transforms every protocol requirement into automated, verifiable actions. Each event triggers the next operational step, eligibility checks, consent status, visit workflows, logistics, data capture, eliminating handoff delays and preventing errors before they reach the site or participant. The Automation Engine enforces BDD-defined rules in real time, ensuring every task runs exactly as the protocol intends. Together, this creates a risk-based operational model that reduces deviations, accelerates cycle times, and keeps complex trials running on schedule with far less oversight.
The event-driven audit log records every action in real time, creating a verifiable trail for compliance and quality oversight. Beyond simply recording changes, it also triggers workflows that maintain data accuracy, protect participant safety, and ensure alignment with regulatory requirements.
Alethium applies Risk-Based Quality Management (RBQM) across every stage of the study. The platform mitigates risk by enforcing protocol rules through Behavior-Driven Development (BDD) and capturing every action as immutable evidence in the event-driven audit log. This approach ensures traceability, compliance, and confidence from startup to submission.
Each record is encrypted at rest with a unique key. If a participant withdraws or when a study closes, the corresponding keys are deleted—rendering all PII data permanently inaccessible. Combined with immutable, time-stamped audit events and strict access controls, this architecture delivers robust protection and verifiable evidence of compliance.

