Accelerating Innovation for Emerging Biopharma

Proven Experience, Exceptional Delivery
Since 2008, clients have relied on us to navigate their most demanding challenges—and we’ve delivered through expertise, trust, and meticulous care. Our long-standing team functions with uncommon cohesion, enabling streamlined communication and consistently exceptional outcomes.
Broad Therapeutic Domains
From vaccines to groundbreaking research in Multiple Sclerosis, Oncology, NASH, Chikungunya, Cardiology, Vaccine/Infectious Diseases, Hepatitis B, and Ophthalmology, Alethium has tackled some of the toughest trials for the world’s leading CROs and sponsors.
Early to Late Stage Trials
Our unified, event-driven CDMS and RBQM foundation ensure the precision required in Phase I, the speed and coordination demanded in Phase II, the scale and multilingual complexity of global Phase III programs, and the reliability essential for Phase IV real-world evidence and follow-up studies.
Proven Scale
Alethium has supported more than 250,000 participants through complex U.S. and international trials. Our unified CDMS and automation-driven workflows deliver the control and insight required to manage global coordination at scale.
DCT and Hybrid Success
Alethium has extensive experience executing multi-arm decentralized and hybrid trials, applying an event-driven CDMS and BDD-defined workflows to manage parallel cohorts, remote procedures, and complex protocol pathways. Our architecture keeps each arm synchronized through unified data capture, automated logistics, and continuous RBQM oversight.
Why Clients Choose Us
Direct Impact
Our clients don’t just use Alethium — they shape it. As an emerging digital-first platform, we integrate client feedback directly into development. Suggestions aren’t deferred to a roadmap; they’re implemented and deployed, keeping every client’s priorities central to how our system evolves.
Know, Like and Trust
Clients want to work with people they know, like, and trust. This principle guides every conversation, decision, and delivery at Alethium. Through strong relationships, we deliver faster, safer, and more reliable studies that are as rewarding to execute as they are successful.
Alacrity
As an agile, results-driven company, Alethium focuses entirely on trial success. Decisions are fast, execution is direct, and our clients never encounter red tape or organizational bloat.
Transparency
Transparency is a core principle at Alethium. From pricing to project execution, we operate with complete clarity. This approach builds trust, reduces risk, and creates lasting partnerships grounded in shared success.

Who We Serve
We serve biopharmas and CROs operating at the intersection of innovation and complexity. Alethium’s digital-first architecture supports decentralized and hybrid trials that require real-time automation, standardized data models, and the ability to execute demanding multi-arm protocols across global study environments.
Value-Driven Biopharma
We amplify the impact of every dollar spent. Alethium is built for emerging biopharmas and CROs working with tight budgets. By combining automation and Behavior-Driven Development, Lean sponsors gain the speed, adaptability, and confidence to run complex trials with the performance and oversight of far larger organizations without the cost or bureaucracy.

What Success Looks Like
A successful study is delivered with precision: timelines met, budgets controlled, and risks proactively mitigated. Alethium’s digital first CDMS ensures operational efficiency and protocol compliance across every workflow, producing verified data that drives confident decisions. For us, success extends beyond technology. It is the consistent reliability, transparency, and partnership that turn first engagements into enduring client relationships.
Where We Excel

Complex and Fast-Paced
Our system is designed for complex multi-arm clinical trials with demanding protocol requirements requiring rapid turnaround times. Protocol-driven automations manage risk, speed onboarding and provide secure actionable data at scale.

Decentralized and Hybrid Trials
Every element of Alethium has been meticulously engineered for decentralized and hybrid clinical trials. Our automation framework, event-driven architecture, and audit log at the core effectively manage complexity, alleviate burdens on participants and sites, and mitigate risks.

Global Reach
Alethium unifies regional requirements into a single compliant automation platform, supporting 400+ languages and regional hosting. Behavior-Driven Development (BDD) preserves protocol fidelity across regions, while encryption and HIPAA, GDPR, and LGPD compliance keep all participant data secure and compliant.
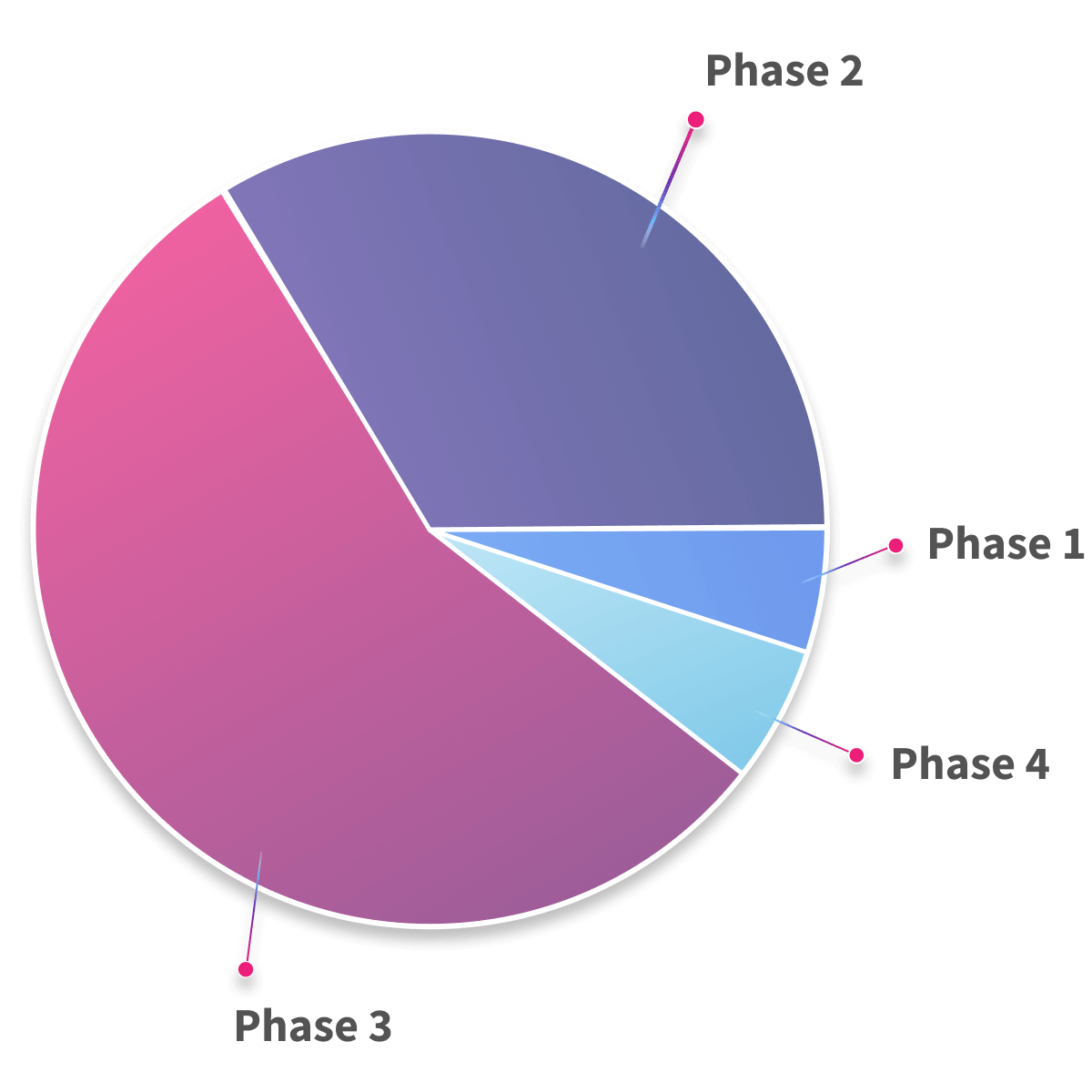
Phase II and Phase III Studies
We partner with emerging biopharmas and CROs running Phase II and Phase III studies, providing the automation, oversight, and scalability required for complex, time-sensitive trials.
Schedule Your Personalized Demo
We listen to your priorities and focus on the risks that matter most to you. Every demo is tailored to your challenges.
FAQs - Common Questions
Alethium supports small to mid-sized biopharma companies and CROs needing an digital-first integrated CDMS with a flexible team for decentralized and hybrid trials.
Alethium provides eConsent, televisits, and remote monitoring tools that reduce site burden and ensure regulatory compliance.
Yes. Alethium’s solutions are designed for small to mid-sized organizations but scale seamlessly to support global studies.
Clients choose Alethium for its digital-first Clinical Data Management System (CDMS), Automation Engine, proven compliance assurance, value-based pricing, and responsive, easy-to-work-with team.
Alethium aligns with FDA 21 CFR Part 11, ICH E6 GCP, HIPAA, GDPR, and LGPD to deliver audit-ready clinical trial operations.

