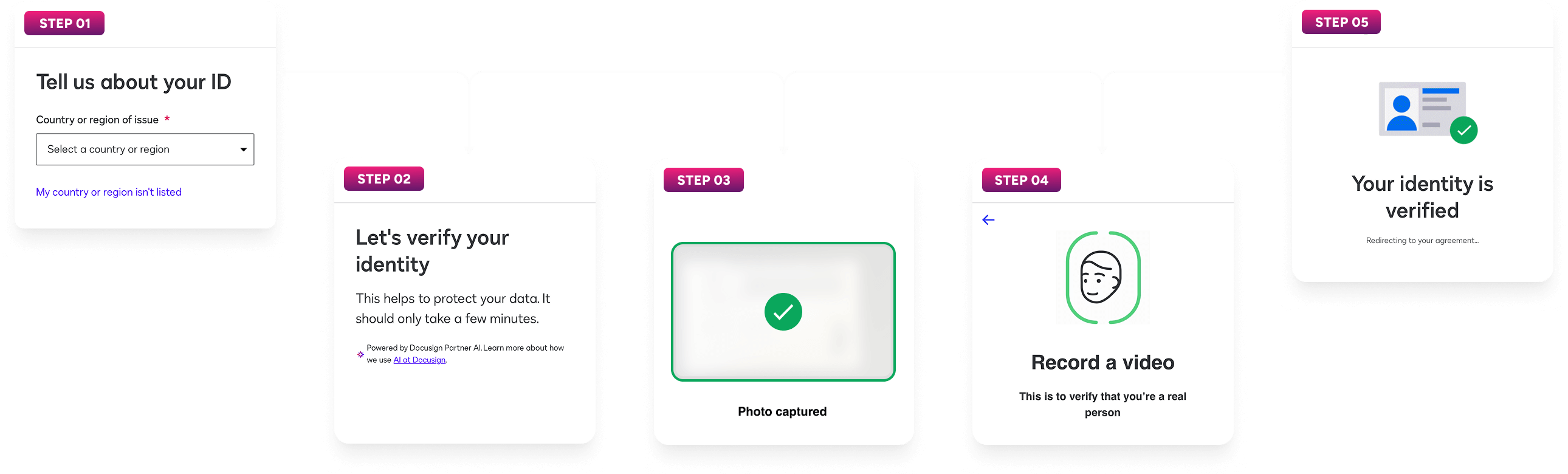
Automated eConsent
Alethium’s digital-first eConsent uses AI-driven ID verification to automate participant onboarding, reduce site workload, and maintain compliance while minimizing drop-off and ensuring faster study startup.

Rapid Onboarding
Fully automated eConsent guides participants through the entire onboarding process in just minutes, minimizing drop-off, enhancing compliance, and allowing them to begin their study journey without delay.
Fewer Protocol Deviations
Automated checks within Alethium’s Risk-Based Quality Management (RBQM) framework verify that participants are properly consented or re-consented before visits, reducing the risk of protocol deviations.
Less Risk, More Control
Integrated within the Automation Engine, eConsent transforms the consent process by cutting site workload, minimizing human error, and enabling faster, safer participant onboarding with built-in compliance.
Audit Confidence
At the heart of Alethium’s CDMS is an event-driven audit log that powers onboarding and consent workflows, delivering complete, real-time traceability for every action and enabling audit readiness and regulatory confidence.
Global Scope
Supports 195+ government IDs and a host of languages including multi-byte and right-to-left scripts.
Built for Complexity
Supports complex consent scenarios with flexible signer configurations, accommodating any number of participants, guardians, or authorized representatives in a single workflow.
De-risking Paper
When paper consent is needed, Alethium always provides the correct ICF and secures the record with deviation protections, an event-driven audit log, and AES-256 encrypted storage.
Manual Options
When manual oversight is required, Alethium supports 21 CFR Part 11 compliant eConsent workflows that maintain complete traceability and secure storage of all study records.
Automated, Secure, Compliant
Automated eConsent protects participants, enforces compliance, and accelerates onboarding for complex, time-critical studies. By combining automation, AI-driven identity verification, and secure storage, Alethium enhances data quality, minimizes deviations, and ensures every ICF remains 21 CFR Part 11 compliant.
Security and Compliance
Compliance
Fully compliant: 21 CFR Part 11, ICH E6 GCP, HIPAA, GDPR, LGPD
Security
SOC 2 Type II, AES-256 encryption and an immutable evert-driven audit log
Regional Hosting
Data residency options include the U.S., EU, and Brazil, ensuring compliance with regional requirements.
Schedule Your Personalized Demo
We listen to your priorities and focus on the risks that matter most to you. Every demo is tailored to your challenges.
FAQs
Automated eConsent streamlines the entire consent process—guiding participants, validating identity, and capturing compliant approvals in minutes. It reduces site workload, lowers drop-off, and improves protocol adherence while producing inspection-ready evidence for every action.
Yes. Each step generates an immutable, time-stamped record in the event-driven audit log. Electronic signatures, versioning, and role-based access provide inspection-ready evidence aligned with 21 CFR Part 11.
Yes. The system supports assenting minors, multiple Legally Authorized Representatives (LARs), and automated re-consent for protocol amendments or ICF updates—keeping records accurate without manual chasing.
Yes. When paper consent is required, Alethium provides the correct ICF and captures it with deviation protections, AES-256 encrypted storage, and complete audit logging. This hybrid capability maintains compliance and audit confidence across all consent methods.
The platform validates government IDs (OCR + security checks), confirms biometric match, and detects liveness to prevent spoofing. Policy-based controls apply higher-assurance steps when needed, enabling secure, compliant eConsent at scale.
Yes. Automated eConsent supports documents from 195+ countries and a host of languages, including multi-byte and right-to-left scripts—enabling seamless global recruitment and onboarding.
Built-in automation verifies that each participant signs the correct ICF and is properly consented (or re-consented) before every visit. This proactive process reduces protocol deviations, saves time, and accelerates study startup.
Alethium’s eConsent isn’t a digital copy of paper. Alethium’s eConsent is built on an event-driven architecture with real-time audit logging, per-record encryption, and Behavior-Driven Development rules. This digital-first design eliminates paper-based delays, enforces protocol adherence automatically, and secures every action.