Televisits From Anywhere on Any Device
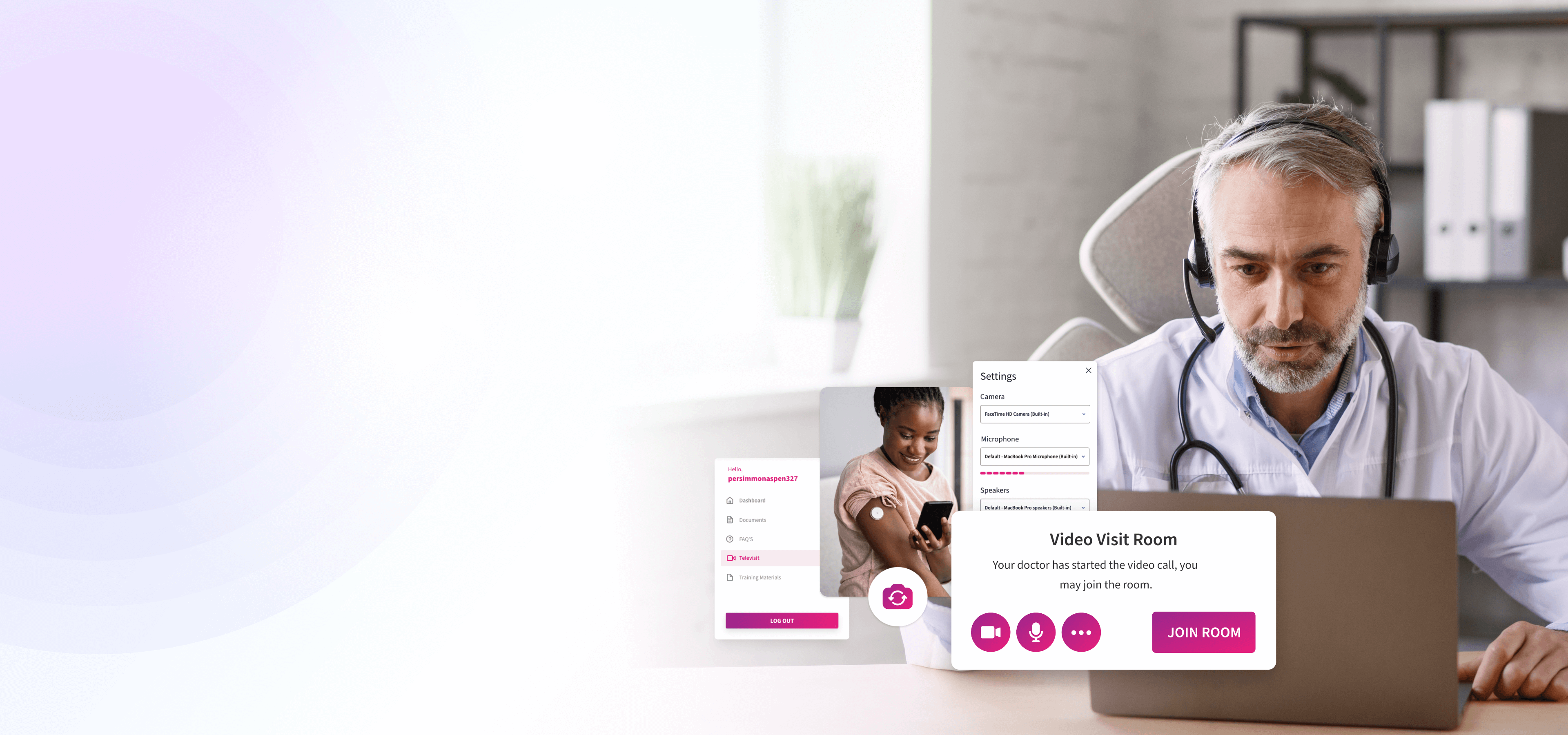
Instant Participant Engagement
Scheduled or ad hoc, televisits launch seamlessly from any device. Each session is authenticated, encrypted end-to-end, and logged in real time to maintain reliable connectivity, regulatory compliance, and full traceability across decentralized and hybrid trials.
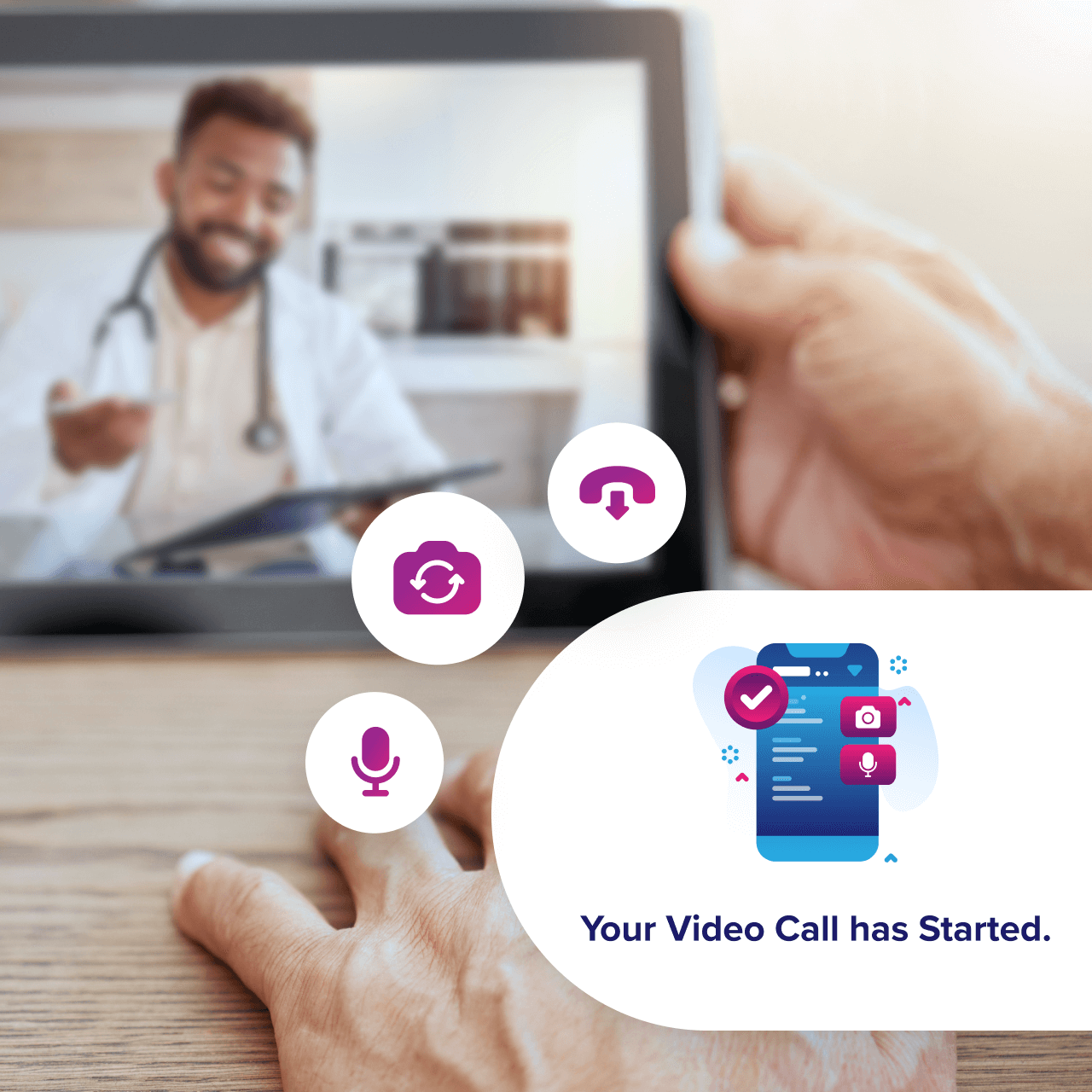
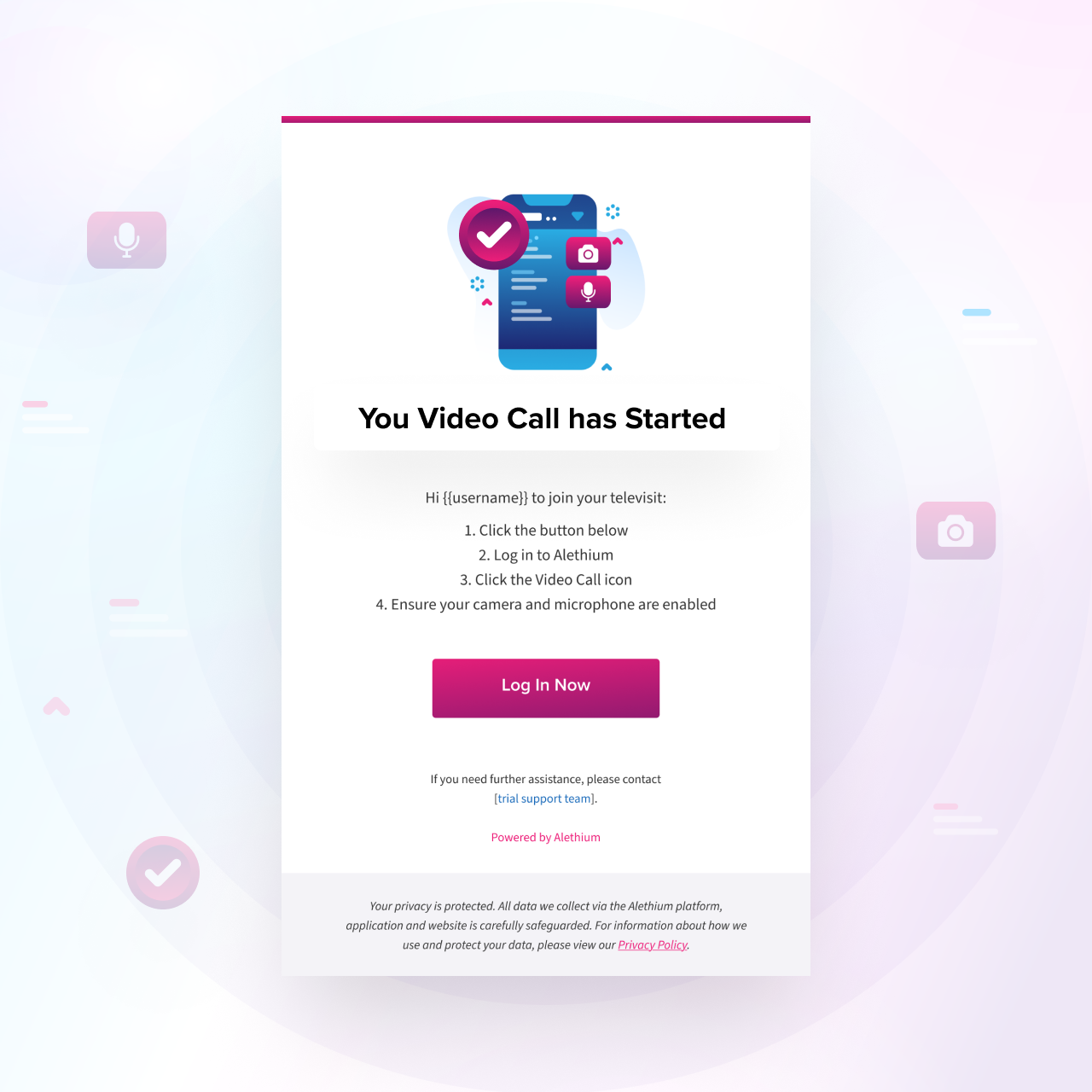
Integrated Scheduling
Televisits automatically synchronize with participant and site calendars and trigger email and SMS reminders to ensure visits occur on schedule and remain fully protocol-compliant.
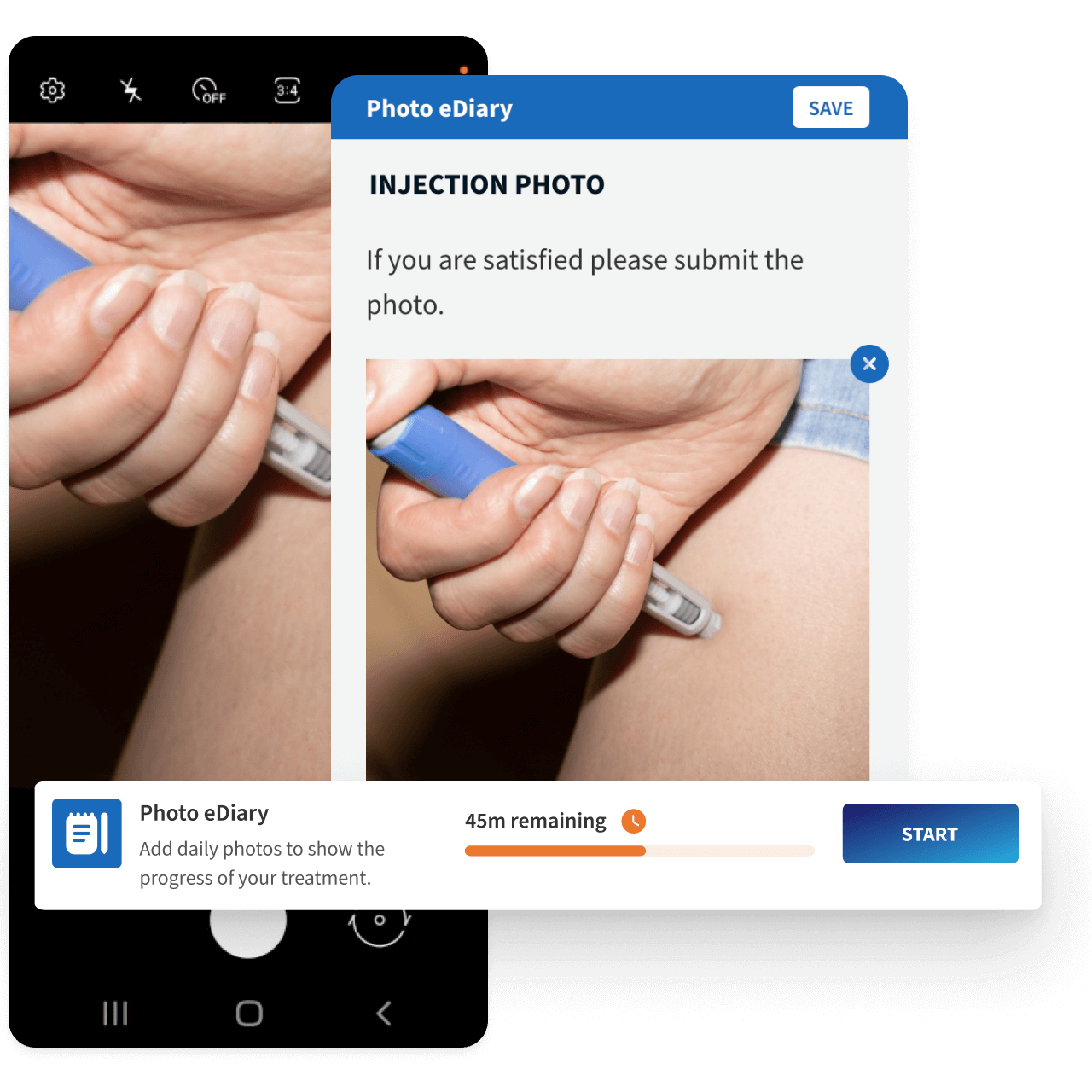
Live Evidence Capture
During televisits, supporting evidence is captured securely, automatically linked to the participant record and visit, stored under validated controls, and logged in the audit trail to maintain end-to-end traceability.
Deviation Protection
As part of Alethium’s Risk-Based Quality Management (RBQM) process, participant consent status is automatically verified before any televisit or on-site visit begins. This proactive control reduces protocol deviations, protects participants, and ensures that all collected data remain valid and regulatory-compliant.
Security and Assurance
Every televisit runs inside Alethium's Clinical Data Platform (CDP). Rigorous safeguards include:
• End-to-end encryption of all media (video and audio)
• Direct peer-to-peer video calls via the established WebRTC standard
• Authenticated APIs for restrict calls to only authorized users
Any Device. Every Protocol. One Standard of Compliance
Televisits within Alethium’s digital-first architecture deliver operational speed, participant continuity, and complete compliance. Behavior-Driven Development (BDD) and event-driven audit logging work together to ensure every televisit is verified, secure, and aligned to protocol, reducing site burden and protecting data integrity across every study.

Schedule Your Personalized Demo
We listen to your priorities and focus on the risks that matter most to you. Every demo is tailored to your challenges.
FAQs - Common Questions
Alethium’s televisits are built on a digital-first, event-driven platform that automates scheduling, consent verification, and documentation. Every interaction is logged, encrypted, and validated in real time through Behavior-Driven Development (BDD). This ensures speed, accuracy, and compliance while removing manual coordination and risk of protocol deviation.
Every televisit runs within Alethium’s fully validated Clinical Data Platform (CDP), ensuring alignment with FDA 21 CFR Part 11, ICH E6 GCP, and HIPAA. All interactions are encrypted end-to-end, recorded in an immutable audit trail, and continuously monitored for compliance and data integrity.
Televisits lower costs, reduce site workload, improve participant retention, and ensure FDA inspection readiness while supporting decentralized and hybrid trial models.
Yes. Alethium’s televisits allow site staff and participants to capture, upload, supporting evidence directly to a participant's records in real time. Each document or photo is encrypted, logged, and validated under controlled access ensuring transparency, traceability, and audit readiness.
Yes. Both participants and site staff can capture photos and source documents during televisits, automatically linking them to the participant record.
Participants connect instantly from any device, no downloads or technical setup required. Automated reminders, integrated scheduling, and secure access links simplify attendance. This frictionless design reduces missed visits, maintains protocol adherence, and improves participant retention across hybrid and decentralized trials.

